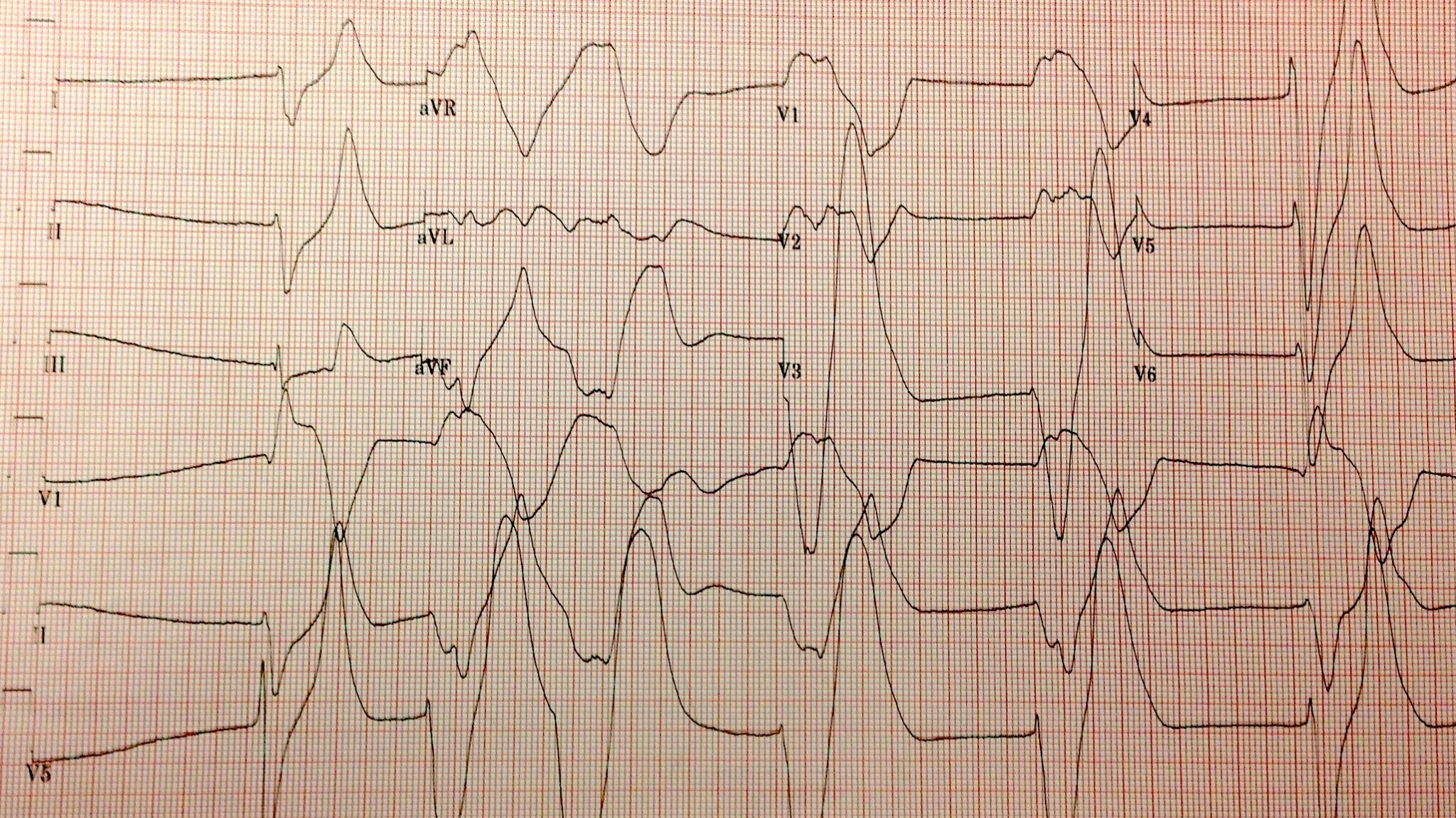
The highest potassium I have ever seen is 9.9 mmol/L…
Don’t worry about that potassium of 9.9, the computer says it’s hemolyzed. twitter.com/kidney_boy/sta…
— Joel Topf (@kidney_boy) April 10, 2013
…however, it was a hemolyzed specimen so it is a tarnished victory. The patient is a dialysis patient in DKA and had a blood sugar of 925 at the time of the hyperkalemia.
 |
| I love that the only things not circled are a creatinine of 8.9 and BUN of 50. |
The patient was started on an insulin drip and one hour later his potassium was 5.2 mmol/L. A drop in the serum potassium of 4.7 mmol/L is profound and atypical. This is due to two factors:
- The initial serum potassium was not that high, despite the subtle EKG changes the real potassium had to be somewhat lower and falsely elevated due to the hemolysis.
- In DKA the hyperkalemia seen on presentation is due to a transcellular shift of potassium from the lack of insulin and increased extracellular osmolality (from the hyperglycemia), both of these are quickly reversible with IV insulin.
K+= 25.4 – (3.02 x pH) + (0.001 x glucose) + (0.028 x Anion Gap)
 |
| Nice picture but this in not an important mechanism in the hyperkalemia of DKA. |
Horacio E Adrogue Funny story about Adrogue, I was eating breakfast at Kidney Week in 2011 when I looked at the name tag of the guy sitting next to me, it was Horacio Adrogue! My chin hit the floor and I started to gush about how much I respected his work and how I loved his NEJM electrolyte reviews and how I was hoping he would autograph my chest and could I pick up his dry cleaning and… then he interrupted me to explain that he was not the Adrogue I was looking for. He was, in fact, The Man’s son and a transplant nephrologist of some regard. How humiliating.
 |
| Nice graph except for micromoles of potassium per mmol of glucose. Really? Could you make it more obtuse? |
One of the interesting conclusions that I learned from the review: one of the most important variables which affects how much the potassium will fall with insulin is the pre-treatments potassium level, the higher the potassium, the greater the response to insulin. The data from that conclusion comes from this study: Serum potassium and acid-base parameters in severe dialysis-associated hyperglycemia treated with insulin therapy. It is an analysis of 43 episodes of hyperglycemia, half DKA and half non-ketotic hyperglycemia. Here is the money shot showing the relationship to initial potassium to drop in potassium:
What would you do for this patient?
I will share the results in a week or so.
Addendum: some of the funnier tweets in response to my original tweet:
@kidney_boy probably artifact. Repeat ECG in am
— Lyle Shehane (@lyleshehane) April 10, 2013
@wanna_be_medic @kidney_boy It’s never good if your ECG looks like it was drawn by a five year old.
— Chump (@bungeechump) April 10, 2013
@kidney_boy unfortunately, it’s intravascular hemolysis.
— Michael Katz (@MGKatz036) April 10, 2013
@kidney_boy have patient follow up in Asystole Clinic in 3-5 days.
— GJ (@GregJNYC) April 11, 2013
We loved that comment so much we made it the Hyperkalemia Merit Badge: