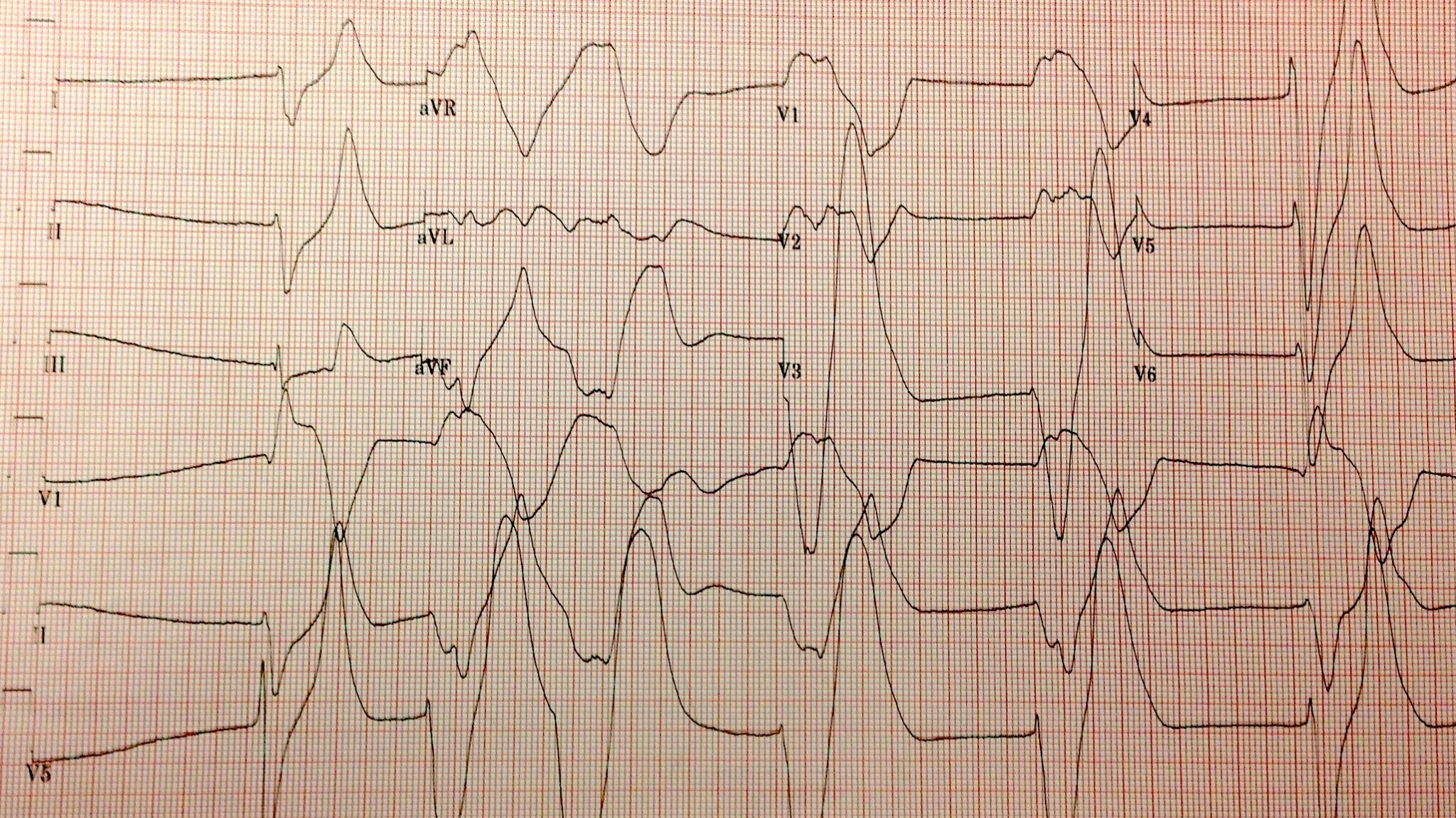
The highest potassium I have ever seen is 9.9 mmol/L…
Don’t worry about that potassium of 9.9, the computer says it’s hemolyzed. twitter.com/kidney_boy/sta…
— Joel Topf (@kidney_boy) April 10, 2013
…however, it was a hemolyzed specimen so it is a tarnished victory. The patient is a dialysis patient in DKA and had a blood sugar of 925 at the time of the hyperkalemia.
 |
| I love that the only things not circled are a creatinine of 8.9 and BUN of 50. |
The patient was started on an insulin drip and one hour later his potassium was 5.2 mmol/L. A drop in the serum potassium of 4.7 mmol/L is profound and atypical. This is due to two factors:
- The initial serum potassium was not that high, despite the subtle EKG changes the real potassium had to be somewhat lower and falsely elevated due to the hemolysis.
- In DKA the hyperkalemia seen on presentation is due to a transcellular shift of potassium from the lack of insulin and increased extracellular osmolality (from the hyperglycemia), both of these are quickly reversible with IV insulin.
K+= 25.4 – (3.02 x pH) + (0.001 x glucose) + (0.028 x Anion Gap)
 |
| Nice picture but this in not an important mechanism in the hyperkalemia of DKA. |
Horacio E Adrogue Funny story about Adrogue, I was eating breakfast at Kidney Week in 2011 when I looked at the name tag of the guy sitting next to me, it was Horacio Adrogue! My chin hit the floor and I started to gush about how much I respected his work and how I loved his NEJM electrolyte reviews and how I was hoping he would autograph my chest and could I pick up his dry cleaning and… then he interrupted me to explain that he was not the Adrogue I was looking for. He was, in fact, The Man’s son and a transplant nephrologist of some regard. How humiliating.
 |
| Nice graph except for micromoles of potassium per mmol of glucose. Really? Could you make it more obtuse? |
One of the interesting conclusions that I learned from the review: one of the most important variables which affects how much the potassium will fall with insulin is the pre-treatments potassium level, the higher the potassium, the greater the response to insulin. The data from that conclusion comes from this study: Serum potassium and acid-base parameters in severe dialysis-associated hyperglycemia treated with insulin therapy. It is an analysis of 43 episodes of hyperglycemia, half DKA and half non-ketotic hyperglycemia. Here is the money shot showing the relationship to initial potassium to drop in potassium:
What would you do for this patient?
I will share the results in a week or so.
Addendum: some of the funnier tweets in response to my original tweet:
@kidney_boy probably artifact. Repeat ECG in am
— Lyle Shehane (@lyleshehane) April 10, 2013
@wanna_be_medic @kidney_boy It’s never good if your ECG looks like it was drawn by a five year old.
— Chump (@bungeechump) April 10, 2013
@kidney_boy unfortunately, it’s intravascular hemolysis.
— Michael Katz (@MGKatz036) April 10, 2013
@kidney_boy have patient follow up in Asystole Clinic in 3-5 days.
— GJ (@GregJNYC) April 11, 2013
We loved that comment so much we made it the Hyperkalemia Merit Badge:






Hi Joel,
I don't buy that all of the hydrogen ions enter the cell accompanied by an anion. The normal concentration of hydrogen ions in the blood is in the nanomolar range. If each ketoacid ion is generated along with a hydrogen ion then substantial buffering must occur to keep the pH above 7. Some of this buffering is provided by bicarbonate (leading to a drop in HCO3 and a concomitant increase in the AG. If all remaining buffering occurred through the mechanism of H+Ketoacid- entry into cells, then the increase in the AG would have to be approximately equal to the fall in bicarbonate.
In fact, what we see is a bicarbonate of 14 (drop of ~10) with an AG of 34. The increase in AG is actually more than that because, with a k of 9, this would add 4 more points to the AG. To maintain electroneutrality in this situation, some of the hydrogen ions have to have moved into the cells in exchange for sodium and potassium (primarily sodium, of course). Otherwise you could not have this extreme imbalance between the increase in the AG and the fall in the bicarbonate.
Gearoid
http://jasn.asnjournals.org/content/18/9/2429.long
Hi Joel
Highest I had was 10.2 (lab sample not an iStat)
Came in with "hyperkalemic paralysis" which I'd never heard of (i'd heard of hypo…) but patient told me he'd had it once before
it was of course 3 hrs before his dialysis was due, so he got a quick trip to dialysis and was right as rain by lunch time.
This doesn't really answer the question. It is true that diagnosing a mixed acidosis in patients with DKA is difficult because the delta/delta is not necessarily reliable. However, even in the extreme cases noted in that review, the ratio was 1.8. In this case, the fall in bicarb is 10 and the increase in AG (including K) is 28 – a ratio of 2.8. Thus, some of the hydrogen ions have to have moved intracellularly unaccompanied by a ketoacid anion. Otherwise, the AG would not have increased to that extent.
Gearoid, I'm convinced. I've got nothing. Thanks for the comments.
Joel is 100% right. Inorganic metabolic acidosis cause a much larger shift of K+ than organic acidosis. The reason for this lies in the presence of monocarboxylate transporters MCT1 and MCT4 which mediates coupled flux of H+ AND organic anion into the cell. For more details, please check out this review article by two giants of renal physiology (Aronson and Giebisch): http://www.ncbi.nlm.nih.gov/pubmed/21980112
I've been thinking about this more and discussed the case with Dr Seifter, our electrolyte/acid-base guru. It turns out that my analysis is not right (of course). What is happening in an organic acidosis is that the anions are generated intracellularly and move outside the cell. To maintain electroneutrality in this setting, the anions either move out with a cation (Na, K or H which is then buffered by the extracellular buffers) or it is exchanged for chloride. In the case above, there has been a dramatic fall in the chloride concentration so most of the AG is caused by a fall in chloride. You are correct about the relatively small increase in K associated with this – most of the anion is accompanied out of the cell by H or exchanged for Cl.
Sorry for going on and on about this, I like to think about these cases and I learned a lot myself…
that was the best explanation of organic acidosis I have ever read. No apology needed.
What are the principle inorganic acids we encounter in clinical medicine? Sulfuric acid from metabolism of Methionine and cysteine? Is respiratory acidosis inorganic? I'd think yes.
This is what didn't make sense to me until JLS explained it very nicely. The organic acid anions are generated intracellularly and so the flow is out of the cell rather than in. They exit the cell in exchange for chloride. If the movement of H+ into the cell was always coupled with the anion, there would be no anion gap as an anion gap presumes that there is some extracellular anion which is associated with a sodium or potassium cation (Na+A- or K+A-). If the only movement of anion was into the cell and it was always accompanied by H+, how could you have an anion gap. The mystery is explained by the fact that the movement is in the other direction.
That is a great paper – however, they too are talking about H+Lactate- entry into muscle cells along that pathway. However, in most organic acidoses, the organic acid is generated intracellularly and there is a marked intracellular acidosis. This actually inhibits potassium efflux from the cell as is mentioned later in that article. Most of the experiments referred to in the article compare the addition of an acid (organic or inorganic) to a bath containing muscle. This simulates and extracellular acidosis which is not what happens in vivo.
In clinical practice, inorganic metabolic acidosis is usually caused by any acidosis which the end result is the production of HCl, i.e. hyperchloremic (non-anion gap) metabolic acidosis. H2SO4, H2PO4- and respiratory acidosis are also considered inorganic.